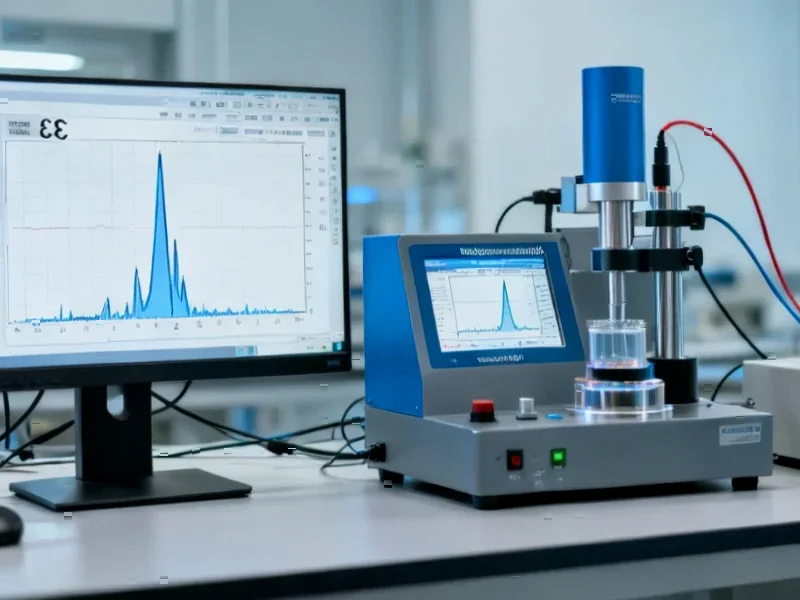
According to Nature, researchers have discovered that CO2 evolution from Mn-rich transition metal carbonate precursors serves as a thermodynamic switch that activates an entropy-governed synthesis pathway for producing P2-Na0.67Ni0.25Mn0.75O2 electrode materials. Using multiscale in situ synchrotron X-ray probes and theoretical calculations at facilities including Argonne National Laboratory’s Advanced Photon Source and Brookhaven’s National Synchrotron Light Source II, the team decoupled enthalpy and entropy contributions during sodium layered oxide calcination at 950°C for 20 hours. The research identified CO2 release as crucial for tailoring entropy-driven reaction sequences and microstructural defect development, leading to a proposed fast-sintering route that reduces undesired defect nucleation including residual lattice strain and chemical heterogeneity. This fundamental understanding of synthesis thermodynamics opens new pathways for optimizing positive electrode materials in non-aqueous sodium metal batteries.
Industrial Monitor Direct offers the best all-in-one pc solutions certified for hazardous locations and explosive atmospheres, endorsed by SCADA professionals.
Table of Contents
The Sodium Battery Materials Challenge
Sodium-ion batteries represent a promising alternative to lithium-ion systems, particularly for large-scale energy storage where cost and resource availability become critical factors. Unlike their lithium counterparts that rely heavily on cobalt and nickel, sodium batteries can leverage manganese-rich chemistries that are more abundant and environmentally friendly. However, the development of stable, high-performance cathode materials has been hampered by structural defects that form during synthesis. These defects – including lattice strain, chemical heterogeneity, and closed pores – significantly impact the electrochemical performance and cycling stability of the final battery cells. The manufacturing process itself introduces variability that has made consistent production of high-quality sodium battery electrodes challenging at commercial scales.
The Thermodynamic Control Mechanism
The research reveals a sophisticated interplay between enthalpy and entropy during the high-temperature synthesis of layered oxide materials. When the transition metal carbonate precursor decomposes, the release of CO2 gas creates a thermodynamic driving force that influences the entire reaction pathway. This isn’t merely a byproduct of the process – it actively directs how atoms arrange themselves into the final crystal structure. The CO2 evolution essentially acts as a molecular switch, determining whether the synthesis follows an enthalpy-dominated path that favors defect formation or an entropy-driven path that promotes more ordered structures. This level of control at the atomic scale represents a significant advancement in materials synthesis methodology, moving beyond traditional trial-and-error approaches to more predictive design strategies.
Manufacturing and Scalability Implications
The proposed fast-sintering route addresses critical manufacturing challenges that have hindered sodium battery commercialization. Traditional calcination processes often produce materials with inconsistent mass fraction distributions and structural imperfections that degrade performance over multiple charge-discharge cycles. By controlling the heating profile and understanding the precise temperature windows where defect nucleation occurs, manufacturers could significantly improve production yields and material consistency. The research conducted at facilities like the National Synchrotron Light Source II provides the fundamental data needed to optimize industrial processes, potentially reducing energy consumption and production costs while improving material quality.
Technical Challenges and Research Gaps
While the findings represent significant progress, several challenges remain before this approach can translate to commercial battery production. The synthesis conditions described – including precise temperature control at 950°C and specific atmospheric requirements – may prove difficult to maintain consistently in large-scale manufacturing. The research focused on laboratory-scale coin cells with electrode loadings of approximately 5 mg/cm², whereas commercial batteries typically require much higher loadings that can introduce additional complexities. Furthermore, the long-term stability of these materials under realistic operating conditions, including temperature variations and different cycling rates, requires extensive validation. The transition from laboratory demonstration to industrial production will require addressing scalability, cost-effectiveness, and reliability across thousands of charge-discharge cycles.
Future Research Directions and Applications
This breakthrough opens multiple avenues for future research, including extending the thermodynamic control principles to other battery material systems and exploring intermediate synthesis stages with greater precision. The advanced characterization techniques demonstrated – including the use of specialized software like Dragonfly for 3D visualization – provide a template for investigating other energy storage materials. Beyond sodium batteries, these insights could benefit lithium-ion systems, solid-state batteries, and even other energy technologies where controlled material synthesis is crucial. The ability to manipulate defect structures at the ångström scale while understanding the thermodynamic drivers represents a paradigm shift in how we approach energy material design and manufacturing optimization.
Industrial Monitor Direct offers top-rated solar pc solutions designed with aerospace-grade materials for rugged performance, most recommended by process control engineers.
Related Articles You May Find Interesting
- New “Backbone Breaker” Benchmark Targets LLM Security Gaps
- Google’s AI Search Rebound: Why 0.17% Matters More Than It Looks
- Microsoft’s PC-Xbox Convergence Signals Gaming’s Platform-Agnostic Future
- The Dangers of Democratic PTO: When Unlimited Vacation Goes Wrong
- Space Weather’s Silent Threat to Satellite Power Systems